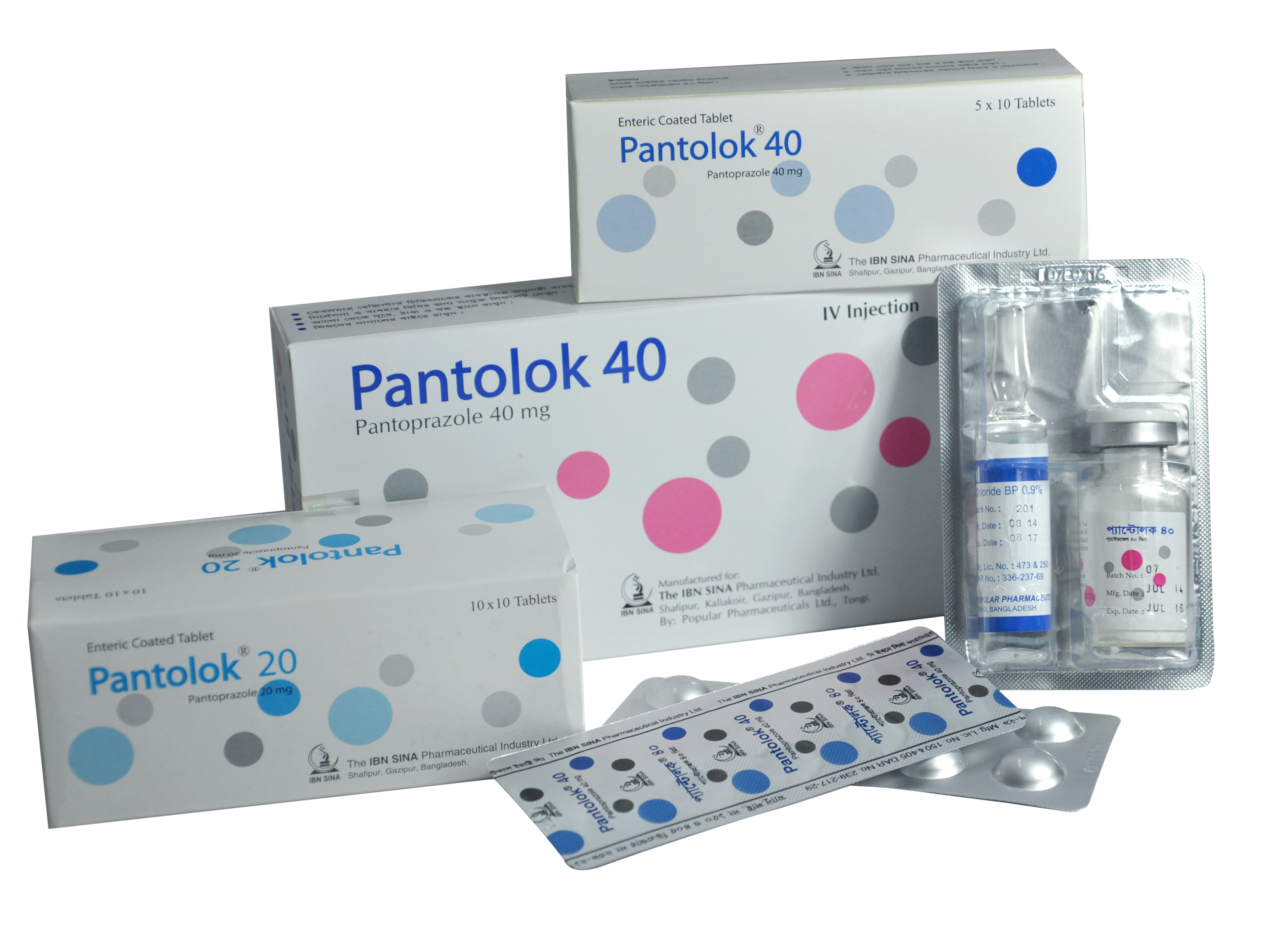
PANTOLOK
PANTOPRAZOLE BP
| NAME | STRENGTH | PACK SIZE | DOSAGE FORM |
|---|---|---|---|
| PANTOLOK 20 MG | 20 MG | 100 S | TABLET |
| PANTOLOK 40 MG | 40 MG | 1 S | INJECTION |
| PANTOLOK 40 MG | 40 MG | 50 S | TABLET |
Pantolok 20 tablet: Each delayed release tablet contains Pantoprazole Sodium Sesquihydrate USP equivalent to Pantoprazole 20 mg. Pantolok 40 tablet: Each delayed release tablet contains Pantoprazole Sodium Sesquihydrate USP equivalent to Pantoprazole 40 mg. Pantolok 40 IV injection: Each vial contains Pantoprazole 40 mg (as lyphollized powder of pantoprazole sodium sesquihydrate BP) and each ampoule contains 10 ml of 0.9% Sodium Chloride Injection BP.
Pantolok is indicated where suppression of acid secretion is of therapeutic benefit. Pantolok is registered for the following indications: - Peptic ulcer diseases (PUD), Gastro esophageal reflux diseases (GERD), Treatment of ulcer resistant to H2 receptor antagonists (H2RAs), Treatment of ulcers induced by non-steroidal anti-inflammatory drugs (NSAIDs), Gastrointestinal (GI) bleeding from stress or acid peptic diseases, Eradication of Helicobacter pylori (in combination with antibiotics), Zollinger-Ellison syndrome, Prophylaxis for acid aspiration syndrome during induction of anesthesia
