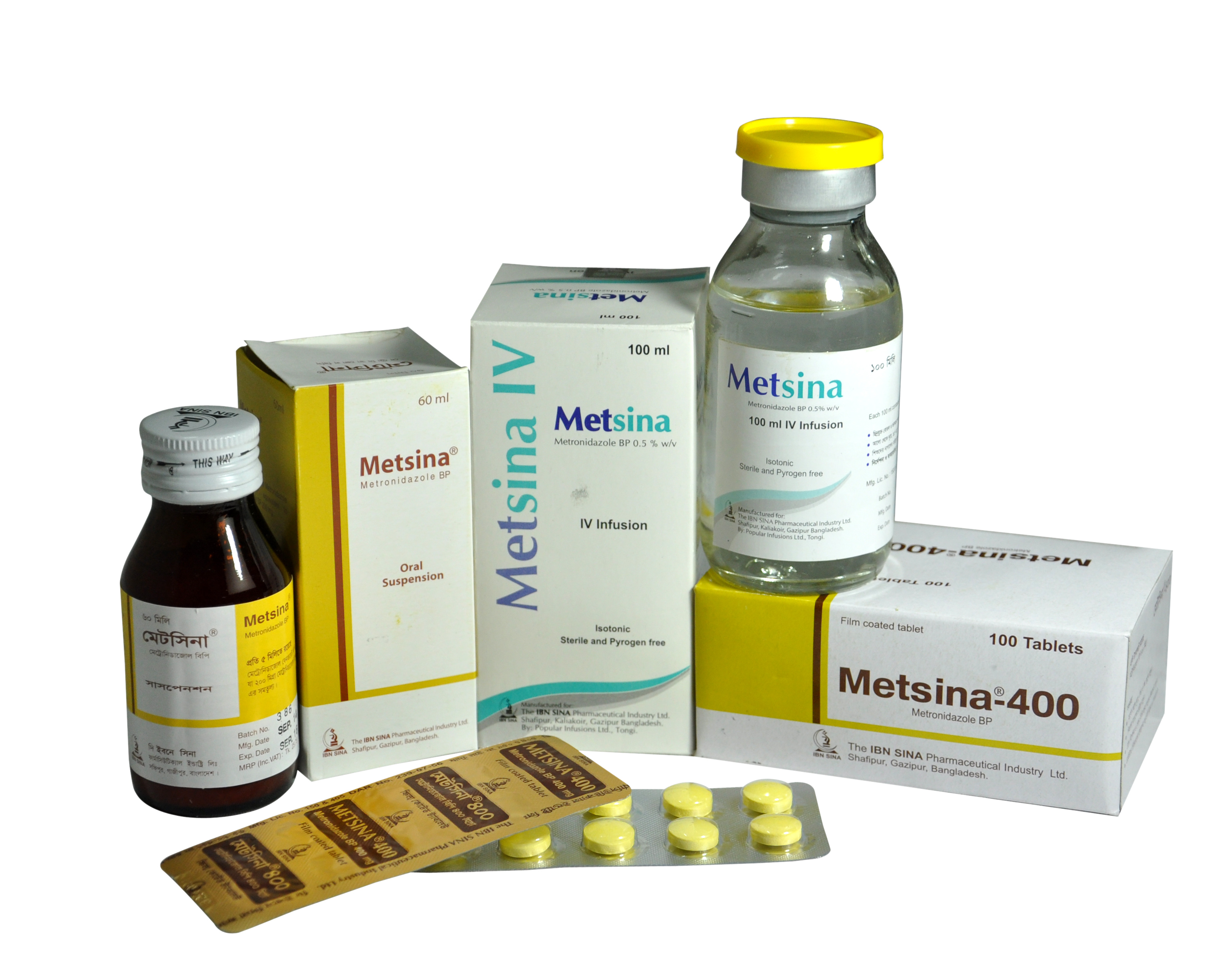
METSINA
METRONIDAZOLE BP
| NAME | STRENGTH | PACK SIZE | DOSAGE FORM |
|---|---|---|---|
| METSINA 100 ML IV INFUSION | 0.5% W/V | 100 ML | IV INFUSION |
| METSINA 200 MG TABLET | 200 MG | 100 S | TABLET |
| METSINA 400 MG TABLET | 400 MG | 100 S | TABLET |
| METSINA 60 ML SUSPENSION | 200 MG/5ML | 60 ML | SUSPENSION |
Metsina Tablet: Each tablet contains Metronidazole BP 200 mg. Metsina 400 Tablet: Each tablet contains Metronidazole BP 400 mg. Metsina Suspension: Each 5 ml contains Metronidazole as Benzoyl Metronidazole BP. Metsina IV infusion: Each 100 ml solution contains Metronidazole BP 500 mg. (5 mg/ ml)
Metsina (Metronidazole) has an extremely broad spectrum antiprotozoal activitiy and iseffective against Trichomonas vaginalis, Entamoeba histolytica, Giardia intestinalis and anaerobic bacteria. Metsina is completely and rapidly absorbed after oral administration. Both unchanged Metronidazole and several metabolites are excreted in various proportions in the urine after oral administration.
All types of Amoebiasis: a) Trichomoniasis (Vaginal and UT infection), Giardiasis, and Gingivitis. b) Anaerobic bacterial infection. c) Leg ulcer and pressure sore..
For the treatment of Amoebiasis 800 mg thrice daily for 5 days, Child: 7-10 years 400 mg, 3-7 years 200 mg tds; 1-3 years 100 mg every 8 hours for 5 days. Giardiasis: 2g daily for 3 days. Child 1-3 years 500 mg daily; 3-7 years 600-800 mg daily; 7-10 years 1g daily for three days. Trichomoniasis: 200 mg 3 times daily for 7 days or 400 mg every 12 hours for 7 days or 800 mg in the morning and 1.2 gm at night for 2 days, or 2g as a single dose. Child: 50 mg 8 hourly (1-3 years), 100 mg 12 hourly (3-7 years), 100 mg 8 hourly (7-10 years) all for 7 days. Anaerobic infection: 400 mg 3 times daily for 7 days. Child: 7.5 mg/kg every 8 hours. Amoebic abscess: 400 mg 3 times daily for 5-10 days. The course may be repeated after 2 weeks if necessary.
Metronidazole is contraindicated for patients with known hypersensitivity to Metronidazole or other Nitroimidazole derivatives.
Metronidazole should not be used in patients with active diseases of CNS and in alcoholism. It is suggested that it should not be given in the first trimester of pregnancy. Also Metronidazole should be used with great care in patients with blood dyscrasias and in liver diseases.
Side effects of Metronidazole include gastrointestinal discomfort, nausea, dryness of mouth, coated tongue, headache, pruritus, skin rash, weakness, unpleasant bitter taste, depression & drowsiness etc. Urethral discomfort, darkening of urine may occur. Temporary and moderate leucopenia and peripheral neuritis may occur as a result of prolonged therapy.
US FDA Pregnancy Category of Metronidazole is B. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed. Metronidazole have been shown to be excreted in human milk. So, caution should be exercised when Metronidazole is administered to a nursing woman.
Disulfiram: Psychotic reactions have been reported in patients who were using metronidazole and disulfiram concurrently. Alcohol: Alcoholic beverages and drugs containing alcohol should not be consumed during therapy and for at least one day afterwards because of the possibility of a disulfiram-like (antabuse effect) reaction (flushing, vomiting, tachycardia). Oral anticoagulant therapy (warfarin type): Potentiation of the anticoagulant effect and increased hemorrhagic risk caused by decreased hepatic catabolism. In case of co-administration, prothrombin time should be more frequently monitored and anticoagulant therapy adjusted during treatment with metronidazole. Lithium: Plasma levels of lithium may be increased by metronidazole. Cyclosporin: Serum cyclosporin and serum creatinine should be closely monitored when co-administration is necessary. Phenytoin or phenobarbital: increased elimination of metronidazole resulting in reduced plasma levels. 5-Fluorouracil: Reduced clearance of 5-fluorouracil resulting in increased toxicity of 5-fluorouracil. Busulfan: Plasma levels of busulfan may be increased by metronidazole, which may lead to severe busulfan toxicity.
Single oral doses of metronidazole, up to 12 g have been reported in suicide attempts and accidental overdoses. Symptoms were limited to vomiting, ataxia and slight disorientation. There is no specific antidote for metronidazole overdosages. In case of suspected massive overdosages, a symptomatic and supportive treatment should be instituted
Store below 30°C. Keep protected from light. Keep medicines out of the reach of children. Do not use later than the date of expiry.
Metsina 400 mg Tablet: Box contains 100 (10x10's) tablets in aluminium strip.
Metsina Tablet: Box contains 100 (10x10's) tablets in aluminium strip.
Metsina Suspension: 60 ml suspension in Bottle. Metsina IV Infusion: Each box contains 1 bottle of 100 ml solution of Metronidazole for intravenous infusion.
